We use cookies to make certain that we supply you with the most effective practical experience on our Web-site. Should you proceed to utilize this site We'll think that you will be satisfied with it.OkPrivacy coverage
Initial things initially. There aren’t any immediate references towards the acronym ALCOA or ALCOA+ in, such as the FDA or EPA GLPs, or within the OECD Principles of fine Laboratory Practice. I don’t believe that it capabilities in almost any of your U.S., EU or Canadian GMPs or GCPs possibly. For that longest time it just sort of appeared5Apparently it initial ‘kind of appeared’ for the reason that Woollen ran outside of room on the slide: “… I do try to remember the consternation of at least just one member with the audience, who in trying to later decipher the “authorities jargon” in my slide, questioned what ALCOA stood for.” right here and there in conference presentations and teaching decks.
Legible data ensures that data can be quickly study and comprehended, stopping misinterpretation.
As organizations consider their forward and reverse offer chain processes and programs utilized to guidance small business functions, it is actually very important that they are in a position to reply all thoughts with regards to traceability as Portion of new serialization specifications established forth in the last a number of several years.
Validating a form field basically implies that the software program checks that you choose to’re not making any obvious problems or omissions. It warns you of These before the data is submitted. Think about the last time you crammed out a signup type online.
In a very Actual physical product including pharmaceuticals or healthcare units, the measure of solution high quality could possibly be in meeting a specification, or in statistical phrases for example the amount of defects for each check here batch.
Just one caveat: your sorts should by no means implement models or the quantity of decimal factors Except Unquestionably in line with the instrument creating the data. Remember, your original data file must often be the initial observation. Rounding is usually a manipulation of the data that may come later on.
21 CFR Section 11 can be a key regulation within the FDA that governs the use of Digital information and Digital signatures during the pharmaceutical industry. It sets forth guidelines to make certain that Digital information are trusted, reputable, and equivalent to paper information.
On the other hand, there may be other concerns you'll want to make to be certain data more info are available within an affordable timeframe.
We’ve witnessed that ALCOACCEA are the core tenets of data high-quality and data integrity, and which they occur straight from the rules.
Being a manufacturer you generate Digital reams of data, so you may speculate which data is most scrutinized. On the whole, regulators look at it essential to focus methods on methods, features, or features that directly:
This white paper demonstrates with examples and charts some great benefits of changeover from the guide, paper centered process to an automated method utilizing a...
Whenever a data point is calculated, promptly file it from the accessible discipline. Make sure that all details demanded by the form or SOP is likewise recorded.
Contemporaneous indicates ‘well timed’. Our memory is risky: the graphic of an observation decays as time goes on. Therefore the more immediately an observation is recorded, the higher the standard of that history. Therefore, data ought to be recorded as They may be observed, along with the document must incorporate a time9I use time below to incorporate both equally time of working day plus the day.
 Luke Perry Then & Now!
Luke Perry Then & Now! Andrea Barber Then & Now!
Andrea Barber Then & Now!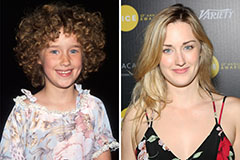 Ashley Johnson Then & Now!
Ashley Johnson Then & Now!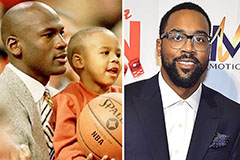 Marcus Jordan Then & Now!
Marcus Jordan Then & Now!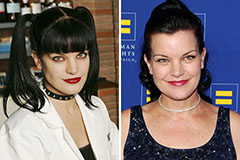 Pauley Perrette Then & Now!
Pauley Perrette Then & Now!